-
PDF
- Split View
-
Views
-
Cite
Cite
Jessica F Hebert, Jess A Millar, Rahul Raghavan, Amie Romney, Jason E Podrabsky, Monique Y Rennie, Allison M Felker, Perrie O’Tierney-Ginn, Mayu Morita, Elizabeth A DuPriest, Terry K Morgan, Male fetal sex affects uteroplacental angiogenesis in growth restriction mouse model, Biology of Reproduction, Volume 104, Issue 4, April 2021, Pages 924–934, https://doi.org/10.1093/biolre/ioab006
Close - Share Icon Share
Abstract
Abnormally increased angiotensin II activity related to maternal angiotensinogen (AGT) genetic variants, or aberrant receptor activation, is associated with small-for-gestational-age babies and abnormal uterine spiral artery remodeling in humans. Our group studies a murine AGT gene titration transgenic (TG; 3-copies of the AGT gene) model, which has a 20% increase in AGT expression mimicking a common human AGT genetic variant (A[−6]G) associated with intrauterine growth restriction (IUGR) and spiral artery pathology. We hypothesized that aberrant maternal AGT expression impacts pregnancy-induced uterine spiral artery angiogenesis in this mouse model leading to IUGR. We controlled for fetal sex and fetal genotype (e.g., only 2-copy wild-type [WT] progeny from WT and TG dams were included). Uteroplacental samples from WT and TG dams from early (days 6.5 and 8.5), mid (d12.5), and late (d16.5) gestation were studied to assess uterine natural killer (uNK) cell phenotypes, decidual metrial triangle angiogenic factors, placental growth and capillary density, placental transcriptomics, and placental nutrient transport. Spiral artery architecture was evaluated at day 16.5 by contrast-perfused three-dimensional microcomputed tomography (3D microCT). Our results suggest that uteroplacental angiogenesis is significantly reduced in TG dams at day 16.5. Males from TG dams are associated with significantly reduced uteroplacental angiogenesis from early to late gestation compared with their female littermates and WT controls. Angiogenesis was not different between fetal sexes from WT dams. We conclude that male fetal sex compounds the pathologic impact of maternal genotype in this mouse model of growth restriction.
Introduction
Poor fetal growth is a common and potentially life-threatening complication of pregnancy [1]. Limited fetal growth may manifest as intrauterine growth restriction (IUGR), a multifactorial disorder characterized by fetal weight below the 10th percentile for gestational age relative to the population [2]. Adverse health outcomes of poor fetal growth include increased perinatal morbidity and mortality with an increased risk of adult-onset diabetes and cardiovascular disease [3–7]. Males seem to be more susceptible to the underlying pathophysiology [8] and long-term health consequences of developmental programming [4–7].
Small babies come from small placentas with gross and histologic features of maternal vascular malperfusion (placental insufficiency), including placental infarctions and accelerated villous maturation [9–14]. This may be related to insufficient delivery of nutrients (e.g., pathologic changes in the uteroplacental arterial network) and/or increased fetoplacental demand (e.g., twin gestations) [14–16]. The reason why IUGR males do more poorly than females is unknown, but it may be related to relatively increased metabolic demands [17]. In addition, male fetal sex has been associated with impaired angiogenesis in murine and porcine models of IUGR [18, 19].
Maternal uterine angiogenesis and pregnancy-induced remodeling are essential for normal pregnancy outcomes in mice and humans [9, 20, 21]. In humans, the uterine arteries (arcuate, radial, and spiral) grow, coil, and dilate in a process related to a combination of angiogenic growth factors (e.g., placental growth factor [PLGF] and vascular endothelial growth factor [VEGF]) and increased blood flow into the intervillous space [22–25]. In mice, the uterine spiral arteries grow de novo during early-to-mid pregnancy, highlighting the importance of this pregnancy-induced process [26].
The uterine vascular network and placental bed (aka. decidua basalis in women and “metrial triangle” in mice) are very similar in mice and women [22, 26–29], but there are large differences in how the uterine and fetoplacental vascular networks interdigitate. The mouse placenta does not have an intervillous space. Instead, it is composed of a labyrinth of interdigitating capillary-like spaces lined by placental trophoblasts encasing fetoplacental capillaries [26]. The decidua in mice and women is composed of uterine natural killer cells (uNK) that are thought to play a vital role in regulating spiral artery angiogenesis. Angiogenic factors like VEGF and PLGF are released by uNK cells to stimulate angiogenesis in the decidua/metrial triangle [28, 29]. Moreover, vascular growth in both the uterus and placenta seems to rely on similar angiogenic/antiangiogenic pathways involved in vasculogenesis (initial development of vessels) and angiogenesis (additional growth of new vessels by branching from existing vessels) to grow capillary-like networks to prune into proper arteries and capillary beds for nutrient exchange [30–32].
We hypothesize that fetal sex may impact uteroplacental angiogenesis, leading to worse clinical outcomes in males compared with females from high-risk pregnancies. To test this, we employed a murine angiotensinogen (AGT) gene titration transgenic (TG) model [33, 34], which was designed to mimic a common human AGT promoter variant (A[−6]G) associated with pregnancy-induced hypertension, IUGR, and abnormal uterine spiral artery remodeling in the first trimester [35–37]. We have previously shown that this TG model has features similar to women with preeclampsia [36] and more recently that their growth-restricted progenies develop adult-onset stress-induced hypertension [34]. An advantage of this model is mice have multiple pups per litter, enabling comparison of fetal sex between siblings with wild-type (WT, 2-copy) genotypes within each litter and between litters relative to maternal genotype (WT vs. TG).
Materials and methods
Transgenic mouse model
Experimental procedures were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University (IP00001507). All procedures were conducted in accordance with ethical guidelines and standards as determined by OHSU IACUC and the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). Angiotensinogen (AGT) 3-copy TG dams (B6.129P2-Agttm1Unc/J) were purchased from The Jackson Laboratory (Bar Harbor, Maine) and backcrossed with WT C57/BL6 mice from Charles River Laboratories (Wilmington, MA) for more than 10 generations before experimentation, similar to our group’s previous work with this model [34, 36]. Adult (11–13 weeks old) TG and WT females were bred with WT males and embryogenesis was timed from the vaginal plug (day 0.5). Fetal sex (SRY) and AGT genotype (3-copy vs. 2-copy) were determined by polymerase chain reaction (PCR) using specific primer sets that yielded products of expected size and sequence using genomic DNA from fetal liver tissue or adult tail snips extracted by DNeasy (QIAgen; Valencia, CA) or REDExtract-n-Amp PCR Reaction Mix (Sigma-Aldrich; St. Louis, MO), respectively, as previously described [34]. Data related to maternal genotype and fetal sex were averaged per litter (≥4 litters/group/experiment).
Tissue samples
For each fetus, the maternal uterine metrial triangles and their corresponding placentae were isolated separately from their siblings to control for fetal sex and fetal genotype. Only WT fetal genotypes (2-copies of AGT gene) were used for all experiments to control for the potential confounding effects of fetal 3-copy AGT expression in TG litters. Intact metrial triangles with attached placentae were evaluated at days 6.5 and 8.5 for uNK activity. Microdissected decidua at day 12.5 was used for angiogenic/antiangiogenic expression analyses. Uterine vascular architecture was evaluated by contrast-perfused three-dimensional microcomputed tomography (3D microCT) imaging at day 16.5. Placental capillary density, transcriptomics, and nutrient transport studies were also evaluated at day 16.5 because of increased confidence of completed pregnancy-induced uterine angiogenesis by this time point [21]. At each gestational age from 6.5 to 16.5, the fetus could be readily identified and excised to provide fetal livers to determine fetal sex and fetal genotype corresponding to its placenta and maternal metrial triangle.
Three-dimensional microCT measurements of uterine arterial structure
Uteroplacental vasculature was perfused with X-ray contrast (Microfil HV-122, Flowtech Inc., Carver, MA) at day 16.5 as previously described [21, 38]. Briefly, perfusion was via a cannulated descending aorta with the use of a perfusion pump while monitoring the exposed uterus to enable selected fill into the uterine arteries and placental labyrinth capillary bed. Three-dimensional microCT images were acquired and reconstructed on a Quantum FX micro-CT (Caliper Life Sciences). Vascular surface renderings were visualized and measured using Amira 3D visualization software. Spiral artery number, branching, and coiling feeding each uteroplacental network were measured independently by two reviewers (MR and JH) blinded to maternal genotype and fetal sex. Averaged values for each fetal sex per each litter per maternal genotype were used for statistical analysis.
Angiogenic/antiangiogenic expression analyses
Protein extracted from microdissected metrial triangles was analyzed using commercially available enzyme-linked immunosorbent assays (ELISAs) (R&D Systems; Minneapolis, MN) for soluble fms-like tyrosine kinase 1 (sFLT-1), VEGF, and PLGF according to manufacturer’s instructions. Each experiment was performed in triplicate, and expression levels were estimated by comparison with kit-provided internal standards. Values were within the dynamic range of each ELISA assay and results for each fetal sex per litter per maternal genotype were used for statistical analysis.
Uterine natural killer cell phenotyping and angiogenesis in whole mounts
Assessment of uNK cell variable phenotype composition and metrial triangle angiogenesis in whole-mount experiments were performed as described by Anne Croy’s group [28, 39]. Briefly, immunofluorescence assays were performed at days 6.5 and 8.5 using in situ uteroplacental whole mounts from TG and WT dams (4–6 litters/group) when uNK cell composition is changing most dramatically [28]. Day 8.5 is also when vasculogenesis/angiogenesis is reportedly at its peak [39]. Uteri from days 6.5 and 8.5 were bisected along the midsagittal line of the mesometrial-antimesometrial axis. They were then stained with FITC-conjugated Dolichos biflorus agglutinin (DBA) lectin (Vector Laboratories; Burlingame, CA) and PE-conjugated antibody against lectin-like receptor Ly49C/I (BD Biosciences; Franklin Lakes, NJ). PE-conjugated anti-CD31 (also known as platelet endothelial cell adhesion molecule [PECAM-1]; BD Biosciences) immunostaining highlighted endothelial cells in the metrial triangle to quantify early angiogenic “blebbing” and pruning “branching metric” described by the Croy group [39]. Wild-type values were compared with those reported by the Croy group [28] for quality control, and only the means per fetal sex per maternal genotype per litter were reported for scientific rigor.
Placental metrics
Placentas corresponding to each 2-copy fetus from WT and TG litters were weighed, measured, and paraffin embedded for stereological assessment of CD31 immunostained histologic sections to calculate fetoplacental labyrinth capillary number and density [40, 41]. Briefly, placentas were cut from a random starting point in thick systematic random sections perpendicular to the chorionic plate. The approximately four thick sections obtained per placenta were mounted into a single block (as described in [40]). Sections 5 μm thick were immunostained for CD31 to highlight endothelial cells outlining fetoplacental capillaries. One histologic section per placental block was evaluated. The placental labyrinth within each section was outlined using Stereo Investigator software (MBF Bioscience; Williston, VT). The number of capillaries within 100% of each placenta’s labyrinth cross-sectional area was calculated using the point-counting method [40, 41] and compared between 4 litters per maternal genotype.
Placental transcriptomics
Complementary DNA (cDNA) libraries were prepared from day 16.5 placentas from male and female 2-copy pups from TG and WT dams using the TruSeq RNA Sample Preparation Kit (Illumina; San Diego, CA) according to manufacturer’s instructions. Purified libraries were quantified on a Bioanalyzer 2100 (Agilent; Santa Clara, CA) using a DNA 1000 chip and sequenced using Illumina HiSeq™ 2000. Reads were cleaned by removing adapters and were filtered by quality (>Q20) and length (>50 bp) using Trimmomatic v0.30 [42]. CLC-workbench (version 9.0; CLCbio) was used to map reads to Mus musculus Genome Reference Consortium Mouse Build 38 (GCA_000001635.6). Transcript abundance and differential gene expression on groups clustered based on PCR analysis were tested for using empirical analysis of DGE in CLC-workbench, controlling false discovery rate at 0.05. Genes that were determined as significantly differentially expressed between/among groups were assigned gene ontology (GO) terms using Database for Annotation, Visualization, and Integrated Discovery (DAVID) [43]. Gene ontology (GO) terms were clustered using REVIGO (medium stringency) [44]. Transcript abundance was reported as RPM averages per fetal sex per maternal genotype per litter (samples from 4 litters/genotype for this—omics pilot study).
Quantitative real-time polymerase chain reaction (qRT-PCR) validation of RNA-sequenced transcriptomics (RNASeq)
Ribonucleic acid from each day 16.5 placental sample was converted to cDNA using SuperScript III First-Strand Synthesis System and amplified using TaqMan probes (Life Technologies; Carlsbad, CA) to measure expression of FLT-1, VEGF, and several genes from key gene ontologies identified by RNASeq relative to Gapdh baseline expression: fatty acid-binding protein 1 and 4 (Fabp1, Fabp4), peroxisomal acyl-coenzyme A oxidase 2 (Acox2), cytochrome c oxidase subunit I and II (Cox1, Cox2), c-type lectin domain family 2 member D (Clec2d), and killer cell lectin-like receptor subfamily B member 1 (Klrb1b) (primers in Supplemental Table 1). Amplification was conducted using a Roche LightCycler as follows: 1 cycle at 95 °C for 5 min, 40 cycles at 95 °C for 15 s, and 65 °C for 60 s (with acquisition at 65 °C). Cycle point crossings were compared with a standard curve for each marker to quantify the relative starting amount of mRNA expressed in each sample.
Placental nutrient transport assays
Placental transport of radiolabeled fatty acids and amino acids were measured in vivo in 4 TG and 4 WT litters at day 16.5 using previously described methods [45–47]. Briefly, 3H-radiolabeled arachidonic acid (2 μCi/kg complexed 1:1 with fatty acid–free albumin) (lipid transport) or 14C-methylaminoisobutyric acid (50 μCi/kg) (amino acid transport) were administered in a 100 μL PBS bolus injection via maternal jugular catheterization, and samples of maternal blood were collected over 4 min from tail vein. After 4 min, placentas and fetuses were collected and weighed. Samples were solubilized with Biosol (PerkinElmer; Waltham, MA) to determine radioactivity in each fraction relative to weight. Maternal-to-fetal unidirectional clearance (K) for each tracer was calculated by dividing fetal counts (N) by the area under the maternal isotope concentration curve (AUC) from time 0 to sacrifice (dpm*min*μl−1) multiplied by placental wet weight in grams (W): K = N/[AUC*W]. Experiments were performed in triplicate and reported as the means per 2-copy fetal genotype (pWT, pTG) per fetal sex per maternal genotype.
Statistical analysis
Fetal sex and maternal genotype were the primary variables for analysis. Within each litter, average (mean) values were calculated for each sex. At least 4 litters per maternal genotype were used in statistical analyses. Data were analyzed by two-way ANOVA with Tukey multiple comparisons post hoc correction when indicated. Results were presented as means ± SEM with significance set as P < 0.05.
Results
Intrauterine growth restriction mouse model
Males from TG dams were smaller than males from WT dams both at day 16.5 (0.48 +/− 0.03 g vs. 0.55 +/− 0.02 g [P = 0.05]) and at birth (1.29 +/− 0.02 g vs. 1.46 +/− 0.04 g [P = 0.02]). Females from TG dams were smaller at birth (1.23 +/− 0.01 g vs. 1.37 +/− 0.02 g [P < 0.001]), but not at day 16.5 (0.48 +/− 0.04 g vs. 0.52 +/− 0.02 g [NS], respectively).
Male fetal sex is associated with reduced spiral artery angiogenesis in TG dams
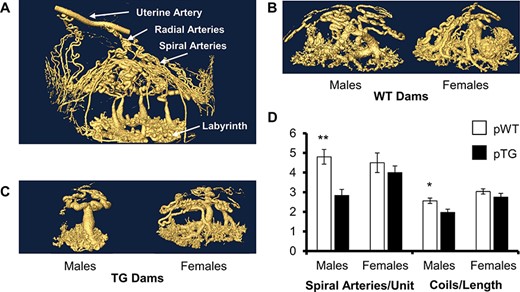
Pregnancy-induced uterine spiral artery angiogenesis measured by 3D microCT imaging was significantly reduced in males from TG dams compared with their female siblings and WT controls (Figure 1). The number of spiral arteries per placenta was halved in male TG fetuses, but unchanged in females, and reduced coiling was also present in male, not female, TG placentas. There was no difference between males and females within WT litters, suggesting that maternal genetic risk was necessary to detect this fetal sex difference.
Uterine spiral artery structure by maternal genotype and fetal sex. (A) Uteroplacental 3D microCT perfusion at day 16.5 shows uterine artery, radial arteries, and the spiral arteries that grow de novo during pregnancy from days 5.5 to 12.5. (B) There is no difference in maternal spiral artery architecture between males and females in WT controls. (C) However, males from TG dams show significantly fewer spiral arteries/placental unit with less spiral artery coiling/length than their female siblings or WT controls (D). 2-copy progeny from 3-copy TG (pTG) dams were used for comparison with 2-copy progeny of WT dams (pWT). Data are the mean ± SEM from 4 litters/group. *P < 0.05, **P < 0.01.
Angiogenic/antiangiogenic levels in maternal decidua (metrial triangles) at midgestation
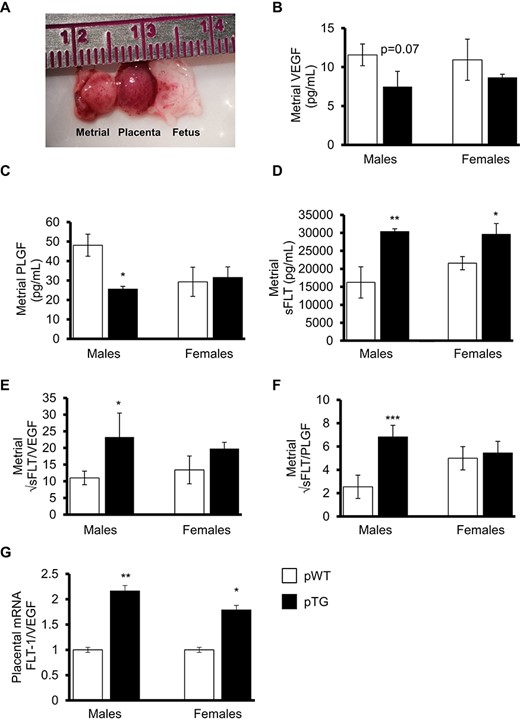
Although peak metrial triangle angiogenesis occurs at day 8.5 in the mouse [40], microdissection of the fetal placenta away from the maternal decidua was not reproducible or reliable until day 12.5 in our hands (confirmed by cytokeratin immunostained histologic analysis of microdissected tissues—data not shown). At day 12.5, the metrial triangles associated with male pups from TG dams showed significantly less PLGF (P < 0.05) and more sFLT-1 (P < 0.01) with a significant shift in the sFLT-1 to PLGF ratio compared with males from WT dams (P < 0.001) (Figure 2). Both fetal sexes from TG dams showed an increase in the placental FLT-1/VEGF expression ratio, although this was more pronounced in pTG males.
Angiogenic and antiangiogenic factors in microdissected metrial triangles or placenta from midgestation. (A) Example of the microdissected metrial triangle, placenta, and fetus at day 12.5. Metrial triangle tissue homogenates were used to measure (B) VEGF, (C) PLGF, and (D) sFLT-1 in WT and TG dams relative to fetal sex and 2-copy (pWT and pTG) fetal genotype. Metrial triangle antiangiogenic sFLT-1 to proangiogenic VEGF (E) and PLGF (F) indices. (G) Relative placental FLT-1/VEGF mRNA expression indices. Data are means ± SEM from 6 litters/group. *P < 0.05, **P < 0.01, ***P < 0.001.
Uterine natural killer cell variable phenotype composition and metrial triangle angiogenesis
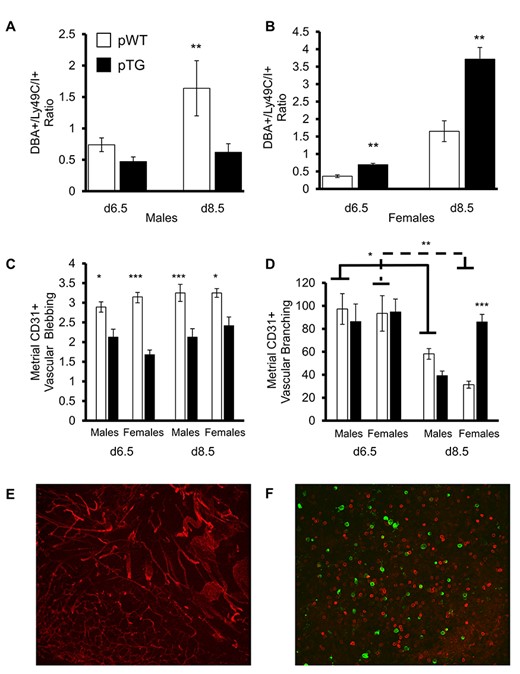
To test whether differences in metrial angiogenesis by fetal sex and maternal genotype could be related to a shift in uNK composition, we evaluated uteroplacental whole mounts at days 6.5 and 8.5 as described previously [28]. Decidual uNK cell composition shift was measured as the ratio of DBA+ to Ly49C/I+ cells per unit area. Higher ratios indicate greater angiogenic signaling since DBA+ cells produce VEGF and PLGF [39]. Metrial triangles supplying male and female fetuses from WT dams both showed an increase in DBA+ uNK cells from days 6.5 to 8.5 (Figure 3A and B), similar to values previously reported by the Croy laboratory [26]. The metrial triangles of TG dams associated with male progeny did not appear to have this similar change in uNK cell phenotype by day 8.5. Metrial triangles of TG dams associated with female progeny had a similar pattern to WT, although there was a greater shift in uNK composition in these TG dams.
uNK cell variable phenotype composition changes, metrial triangle angiogenesis, and vascular pruning in early gestation. uNK cell composition in metrial triangles expressed as the ratio of DBA+ to Ly49C/I+ cells per unit area by male (A) and female (B) fetal sex controlling for fetal genotype (2-copy only) and maternal genotype (WT and TG) revealed the expected increase in proangiogenic uNK cells by day 8.5 compared with day 6.5 in both sexes from WT dams. Males from TG dams failed to show this increase. However, females from TG dams had more DBA+ uNK cells than females from WT dams. (C) CD31 positive endothelial “blebbing,” which is an indicator of early angiogenesis, was more common in the metrial triangles of WT dams, independent of fetal sex. (D) Vascular branching/unit area reduced as the angiogenic networks were pruned into proper arteries. Females from TG dams had significantly less overall pruning with more residual branching at day 8.5. Data are the means ± SEM of 4 litters/group. *P < 0.05, **P < 0.01, ***P < 0.001. (E) Representative staining of blood vessels (CD31, red) in a day 6.5 female TG mouse at 20x magnification. (F) Representative staining of DBA+ (green) and Ly49C/I+ (red) uNK cells in a day 6.5 female TG mouse at 20x magnification.
To test for differences in endothelial “blebbing” and vascular pruning [32, 39] in the metrial triangle by fetal sex and maternal genotype, we stained whole mounts for the endothelial marker CD31 and measured them as described by the Croy laboratory [28, 39]. We observed less vascular blebbing in all metrial triangles of TG dams compared with WT controls independent of fetal sex at days 6.5 and 8.5 (Figure 3C). Vascular pruning from days 6.5 to 8.5 led to fewer branches/area in all metrial triangles from both sexes and both maternal genotypes (Figure 3D). However, males from TG dams showed more pruning than their female siblings. Therefore, although both fetal sexes from TG dams had reduced angiogenic blebbing, pTG females did not prune as vigorously as their pTG male siblings. Examples of CD31 staining and DBA+/Ly49C/I+ staining in a whole-mount section can be found in Figure 3E and F, respectively.
Placental metrics
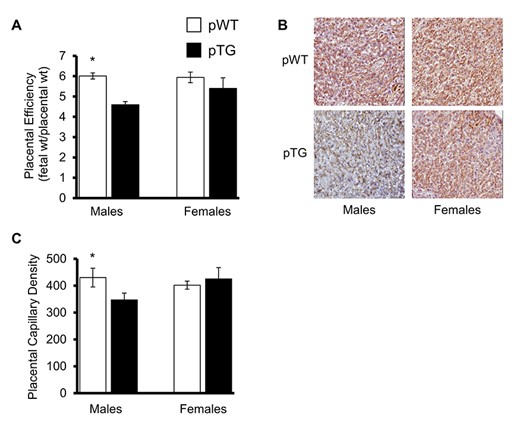
Placental weights were not statistically different between pTG and pWT groups at day 16.5, but pTG males had a significantly lower fetal:placental weight ratio (index of placental efficiency) compared with controls (P < 0.05) and their female siblings (Figure 4A). Placental stereometric analysis of CD31 immunostained sections (Figure 4B) revealed fewer capillaries per placental labyrinth cross-sectional area in pTG males compared with their pTG female siblings and WT controls (Figure 4C).
Placental efficiency and capillary density. (A) Placental efficiency, which is the ratio of fetal weight to placental weight, was significantly reduced in males from TG dams compared with their female siblings and WT controls. Stereometric analysis of CD31 immunostained placental histologic sections at day 16.5 (B) revealed that placental capillary density was also reduced in male placentas from TG dams (C). Data are the means ± SEM of 4 litters/group. *P < 0.05, **P < 0.01, ***P < 0.001.
Placental transcriptomic analysis and placental nutrient transport
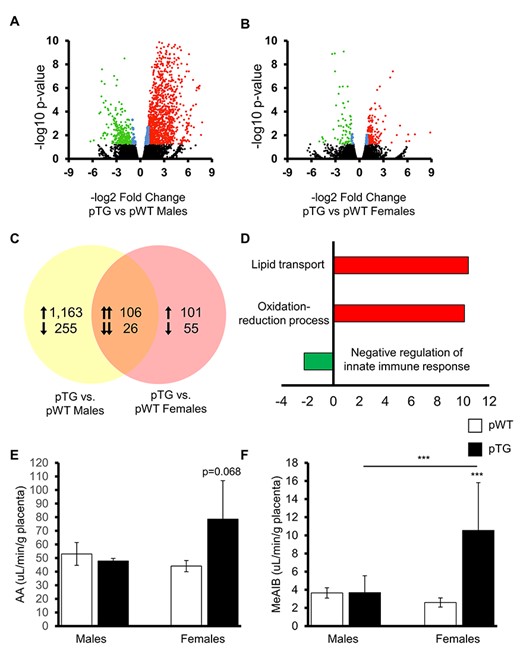
To investigate whether there are differences in placental expression at day 16.5 by fetal sex and maternal genotype, we employed an exploratory placental transcriptomic approach. This time point was chosen because of reproducible microdissection of placental labyrinths away from maternal metrial triangles. Males from TG dams had significantly higher abundances of gene transcripts than WT controls (Figure 5A). There were only minimal differences in gene expression abundance between females from TG and WT dams (Figure 5B). Overall, 132 genes were similar in their expression between males and females from TG dams compared with matched WT controls (Figure 5C).
Placental transcriptomics and placental nutrient transport. Volcano plots showing expression differences for (A) male and (B) female (2-copy) progeny of TG dams compared with sex-matched (2-copy) progeny of WT dams revealed significant transcript differences at day 16.5 with more upregulation and downregulation of placental genes expressed by pTG males compared with controls. (C) pTG males and females shared ~132 genes that had differential transcript abundances compared with age and sex-matched pWT controls, which when classified according to ontogeny (D) indicated enrichment as upregulation (red) of lipid metabolism and oxidative stress pathways with downregulation (green) of the negative regulation of the innate immune response. The x-axis represents a scale of fold change (positive and negative) in transcript abundance between groups. Placental lipid transport measured by radio-labeled arachidonic acid (E) and amino acid transport measured by methylaminoisobutyrate (F) showed slightly increased placental nutrient transport in pTG female progeny at day 16.5. Data are the means +/− SEM from 4 litters/group. *P < 0.05.
After performing GO enrichment for gene lists that were significantly different in abundance between groups (higher or lower expression than controls), we found the similar genes in pTGs were involved in the upregulation of lipid transport pathways, upregulation of oxidation-reduction, and downregulation of negative regulators of the innate immune response (Figure 5D). Validation of candidate genes within these pathways by qRT-PCR correlated well with patterns observed in RNASeq data (overall R2 = 0.98, P < 0.001). Notably, expression of both Clec2d and Klrb1b was significantly downregulated in the placentas of pTG males compared to WT males. Klrb1b is also known as Cd161 and is expressed by NK cells; in particular, it is linked to regulating and reducing NK cell cytotoxicity [48]. Protein KLRB1 binds to lectin-like transcript-1 (LLT1), which downregulates NK-mediated lysis; LLT1 is encoded by Clec2d [49]. Pathway analysis and validation of the differentially regulated genes between pTG male and female placentas is the subject of an ongoing investigation by our group. Placental nutrient transport assays at day 16.5 showed greater transport in pTG females compared with their male siblings (Figure 5E and F), which may represent a placental compensatory mechanism to the TG maternal phenotype.
Discussion
Our data suggest that fetal sex may compound maternal high-risk genotypes/phenotypes, leading to abnormal uterine spiral artery angiogenesis and the cascade of events culminating in compromised fetal growth. This is an important observation because male babies have an increased risk of perinatal morbidity/mortality; they are more susceptible to long-term developmental programming of adult-onset diseases [4, 8, 50]. Although male fetal sex vulnerability is well-described, the mechanism is poorly understood.
Poor fetal growth is a multifactorial syndrome, and our model focuses on two risk factors: maternal genotype and fetal male sex. The maternal genetic high-risk mouse model mimics the 20% higher plasma AGT levels observed in humans with the A-6 AGT promoter variant [33, 35, 51, 52]. This genetic variant is a common allele present in approximately 14% of Caucasians and imparts a significantly increased risk of IUGR associated with spiral artery pathology compared with the G-6 allele [37, 51]. In this study, we controlled for the fetal genotype by restricting analysis to 2-copy (WT) mice from TG dams to isolate the effects of fetal sex and maternal genotype in this model. Future studies will explore the impact of fetal genotype on outcomes.
We suspect male fetal sex may contribute to poor fetal growth because it appears to impact uNK composition in the uterine lining (decidua; metrial triangle) and uteroplacental angiogenesis. uNK cells are abundant in both murine and human decidua during pregnancy and are characterized as Ly49, DBA+/−, in the mouse [53]. DBA+ cells peak around days 8.5–10.5 and release proangiogenic factors like VEGF and PLGF [54]. Although we did not see a difference in placental invasion in the metrial triangles studied at days 12.5 and 16.5 in the model (data not shown), we cannot exclude differences in pTG male placental cell interactions with maternal uNK cells compared with controls. However, we think direct cell-to-cell interaction may not be necessary because spiral artery angiogenesis is complete by midgestation in mice before the placenta invades into the metrial triangle [55]. In turn, we and others are exploring the possibility that placental exosome paracrine/endocrine signaling may play a role in this process [56].
Our exploratory placental transcriptomic study suggested that maternal genotype and fetal sex may impact placental nutrient transport. We hypothesized that pTG male placentas would be less efficient and transport fewer nutrients to the male fetus compared with their female littermates and controls. This was reasonable because we observed a decreased fetal:placental ratio in pTG males, but not females. We were surprised to learn that pTG placentas upregulate nutrient transport genes by day 16.5 and pTG females show significantly increased amino acid transport compared with their siblings and controls. Perhaps it is not unexpected that the placentas in TG dams transport more nutrients late in gestation compared with WT controls, despite lower birthweight. Sheep studies have shown that maternal nutrient restriction at midgestation leads to compensatory increases in nutrient transport and placental size by term [57]. Therefore, we now suspect that the change in placental transport observed in our study near term (day 16.5) may be compensating for relative placental insufficiency earlier in gestation and that this compensation may be more effective in pTG females compared with their male siblings. Another recent transcriptomics study using human placentas from first-term pregnancies indicates that males may impact extravillous trophoblast function related to uteroplacental interface microenvironment, thus inhibiting spiral artery invasion and remodeling in human pregnancies [58]. Comparing placental expression profiles and nutrient transport earlier in gestation (e.g., days 8.5, 10.5, 12.5) will be needed to explore this hypothesis.
In summary, we tested for fetal sex effects on uteroplacental angiogenesis at early (d6.5, d8.5), mid (d12.5), and late (d16.5) gestation in a mouse model of fetal growth restriction. Males from TG dams showed significant differences compared with their female siblings and WT controls at each stage of uterine spiral artery angiogenesis from days 6.5 to 16.5. We observed fewer DBA+ uNK cells at days 6.5 and 8.5 with lower levels of proangiogenic factors (VEGF, PLGF) and greater antiangiogenic sFLT-1 in the metrial triangles of pTG males. The consequence was less angiogenic blebbing, relatively greater pruning of these angiogenic networks, and significantly fewer spiral artery branches and coils by day 16.5. The impact of this altered maternal uterine vascular geometry on blood flow is only beginning to be modeled in reliable in vivo uteroplacental studies [19, 59]. However, one would expect that having fewer, straighter spiral arteries feeding a placenta would increase blood flow velocity, leading to greater shear stress and turbulence [60], possibly increasing damage to the placenta and IUGR or activating endothelial cells differently than in vessels with more laminar flow. Male placentas from TG dams also expressed more FLT-1 and less VEGF mRNA compared with sex-matched WT controls, which were associated with fewer placental capillaries. This also likely contributes to poor fetal growth.
Together, our data provide a potential mechanism that may explain excessive vulnerability of males compared with females during fetal growth and development. In those exposed to maternal risk factors like maternal genotype in this model, reduced uteroplacental angiogenesis in males without compensatory increases in placental transport observed in females may result in fetal growth restriction.
Conference presentation
Society of Reproductive Investigation, March 7–11, 2019, Paris, France.
Acknowledgments
We appreciate the generosity of Dr. Anne Croy for her contributions to our understanding of uNK cells and their important role in maternal-mediated uterine spiral artery angiogenesis in the mouse.
Several authors have changed institutions since this work was completed and entered the manuscript stage. JFH is in the Department of Anesthesiology and Perioperative Medicine at Oregon Health & Science University. JAM is in the Department of Computational Medicine and Bioinformatics at the University of Michigan. AR is in the Department of Biology at Portland State University and the School of Veterinary Medicine at UC Davis. MYR is the Director of Scientific Affairs for MolecuLight, Inc. AF is in the Department of Pathology and Molecular Medicine at McMaster University. RR is in the Department of Biology at The University of Texas at San Antonio.
Conflicts of Interest
The authors have declared that no known conflicts of interest exist.
Footnotes
†Grant Support: This study was funded by the National Institute of Child Health and Human Development (1R21HD068896-01A1), the Office of Women’s Health Research: Oregon K12 BIRCWH (HD043488-08), the Society of Reproductive Investigation, and the Oregon Medical Research Foundation.